Chemical Reactions - Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
Chemical Reactions - Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.: Overview
This topic covers various concepts like Half Life Period, , etc.
Important Questions on Chemical Reactions - Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
The rate of a reaction escalates four times when the temperature changes from to Determine the energy of activation of the reaction, assuming that it does not change with temperature?
The activation energy for the reaction is at . The fraction of molecules having energy equal to or greater than activation energy is:
In a pseudo first order hydrolysis of ester in water, the following results are obtained:
| in seconds | ||||
(i) The average rate of reaction between the time interval 30 to 60 seconds and
(ii) The pseudo first order rate constant for the hydrolysis of ester is?
For a decomposition reaction the values of rate constant k at two different temperatures are given below :
The value of activation energy for this reaction is:
A first order reaction is complete in minute. Calculate the time taken for the reaction to be complete:
,
The half-life for decay of radioactive is 5730 years. An archaeological artifact containing wood has only 80% of the activity as found in living trees. The age of the artifact would be:
[Given: log 1.25 = 0.0969]
The ratio of disappearance of B is The rate of reaction and rate of change in concentration of A and C would be:
For a hypothetical reaction , the activation energy for forward and backward reactions are and respectively. The heat of reaction is
In a first-order reaction, the concentration of reactant decreases from in. The rate constant of reaction in is
Consider a reaction . When concentration of both the reactants G and H is doubled, the rate increases by eight times. However, when concentration of G is doubled keeping the concentration of H constant, the rate is doubled. The overall order of the reaction is:
Consider a reaction . When concentration of both the reactants and is doubled, the rate increases by eight times. However, when the concentration of is doubled, keeping the concentration of constant, the rate is doubled. The overall order of the reaction is:
Consider the chemical reaction:
The rate of this reaction can be expressed in terms of time derivatives of concentration of Identify the correct relationship amongst the rate expressions:
The quantitative estimation of change in the rate of reaction with change in concentration does not always follow stoichiometric equation. Justify.
For a reaction is in , what is the average rate of reaction?
If for the formation of from and is , then for decomposition of mole of is _____.
Enter your correct answe as A, B or C.
In the reaction , if is increased by three times then the difference in the rate is _____ of the initial rate.
From the following data
Which of the following is the appropriate of the reaction?
What is the order of a reaction whose rate constant is ?
The plot of versus for a reversible reaction is shown.
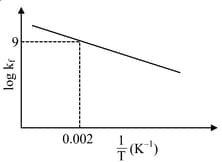
Pre-exponential factors for the forward and backward reactions are and , respectively. If the value of for the reaction at is , the value of at is
equilibrium constant of the reaction, rate constant of forward reaction, rate constant of backward reaction]
At , the half life for the decomposition of is and is independent of the initial concentration of . The time required for of the to decompose is (Given: )
